what do animals ranging from corals to humans have in common?
What are Coral Reefs
Actualization as solitary forms in the fossil record more than 400 million years ago, corals are extremely ancient animals that evolved into modernistic reef-building forms over the last 25 million years. Coral reefs are unique (eastward.thousand., the largest structures on earth of biological origin) and circuitous systems. Rivaling old growth forests in longevity of their ecological communities, well-developed reefs reflect thousands of years of history (Turgeon and Asch, in press).
Corals and their Kind
Corals are anthozoans, the largest form of organisms within the phylum Cnidaria. Comprising over half dozen,000 known species, anthozoans too include bounding main fans, ocean pansies and anemones. Stony corals (scleractinians) make upwards the largest order of anthozoans, and are the group primarily responsible for laying the foundations of, and building upwards, reef structures. For the most function, scleractinians are colonial organisms composed of hundreds to hundreds of thousands of individuals, chosen polyps (Barnes, R.D., 1987; Lalli and Parsons, 1995).

As members of the phylum Cnidaria, corals have only a limited caste of organ development. Each polyp consists of iii basic tissue layers: an outer epidermis, an inner layer of cells lining the gastrovascular cavity which acts as an internal infinite for digestion, and a layer called the mesoglea in between (Barnes, R.D., 1987).
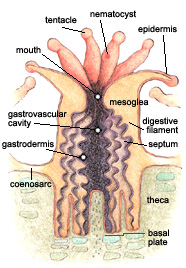
All coral polyps share 2 bones structural features with other members of their phylum. The showtime is a gastrovascular cavity that opens at merely i end. At the opening to this crenel, commonly chosen the mouth, food is consumed and some waste products are expelled. A second characteristic all corals possess is a circle of tentacles, extensions of the torso wall that surround the oral cavity. Tentacles aid the coral to capture and ingest plankton for food, clear away debris from the oral cavity, and act as the animal's primary means of defense (Barnes, R.D., 1987; Levinton, 1995).
While coral polyps have structurally simple body plans, they possess several distinctive cellular structures. 1 of these is called a cnidocyte—a type of jail cell unique to, and characteristic of, all cnidarians. Constitute throughout the tentacles and epidermis, cnidocytes contain organelles chosen cnidae, which include nematocysts, a blazon of stinging cell. Because nematocytes are capable of delivering powerful, often lethal toxins, they are essential to capturing casualty, and facilitate coralline agonistic interactions (Barnes, R.D., 1987).
About corals, like other cnidarians, incorporate a symbiotic algae chosen zooxanthellae, inside their gastrodermal cells. The coral provides the algae with a protected environment and the compounds necessary for photosynthesis. These include carbon dioxide, produced past coral respiration, and inorganic nutrients such as nitrates, and phosphates, which are metabolic waste products of the coral. In render, the algae produce oxygen and help the coral to remove wastes. Virtually chiefly, they supply the coral with organic products of photosynthesis. These compounds, including glucose, glycerol, and amino acids, are utilized by the coral every bit building blocks in the manufacture of proteins, fats, and carbohydrates, as well as the synthesis of calcium carbonate (CaCOiii). The mutual commutation of algal photosynthates and cnidarian metabolites is the cardinal to the prodigious biological productivity and limestone-secreting capacity of reef building corals (Barnes, R.D., 1987; Barnes, R.S.K. and Hughes, 1999; Lalli and Parsons, 1995; Levinton, 1995; Sumich, 1996).

Zooxanthellae often are critical elements in the continuing wellness of reef-building corals. As much as 90% of the organic material they manufacture photosynthetically is transferred to the host coral tissue (Sumich, 1996). If these algal cells are expelled by the polyps, which tin occur if the colony undergoes prolonged physiological stress, the host may die shortly afterwards. The symbiotic zooxanthellae likewise confers its color to the polyp. If the zooxanthellae are expelled, the colony takes on a stark white appearance, which is usually described as "coral bleaching" (Barnes, R.S.K. and Hughes, 1999; Lalli and Parsons, 1995).
(top)
From Polyp to Reef
Massive reef structures are formed when each stony coral polyp secretes a skeleton of CaCOiii. Most stony corals take very small polyps, averaging 1 to iii mm in diameter, but entire colonies can grow very big and counterbalance several tons. Although all corals secrete CaCO3, not all are reef builders. Some corals, such every bit Fungia sp., are alone and take single polyps that tin can grow as big equally 25 cm in diameter. Other coral species are incapable of producing sufficient quantities of CaCO3 to form reefs. Many of these corals do not rely on the algal metabolites produced past zooxanthellae, and live in deeper and/or colder waters across the geographic range of almost reef systems (Barnes, R.D., 1987; Sumich, 1996).

The skeletons of stony corals are secreted by the lower portion of the polyp. This process produces a cup, called the calyx, in which the polyp sits. The walls surrounding the cup are called the theca, and the floor is called the basal plate. Thin, calcareous septa (sclerosepta), which provide structural integrity, protection, and an increased surface area for the polyp'due south soft tissues, extend upward from the basal plate and radiate outward from its eye. Periodically, a polyp volition lift off its base and secrete a new flooring to its cup, forming a new basal plate above the old one. This creates a minute sleeping accommodation in the skeleton. While the colony is live, CaCO3 is deposited, adding partitions and elevating the coral. When polyps are physically stressed, they contract into the calyx so that virtually no part is exposed above the skeletal platform. This protects the organism from predators and the elements (Barnes, R.D., 1987; Sumich, 1996).
At other times, the polyp extends out of the calyx. The timing and extent to which a polyp extends from its protective skeleton often depends on the time of the day, as well as the species of coral. Most polyps extend themselves furthest when they feed on plankton at night.
In addition to a substantial horizontal component, the polyps of colonial corals are continued laterally to their neighbors by a thin horizontal sheet of tissue called the coenosarc, which covers the limestone between the calyxes. Together, polyps and coenosarc found a sparse layer of living tissue over the cake of limestone they have secreted. Thus, the living colony lies entirely higher up the skeleton (Barnes, R.S.K. and Hughes, 1999).

Colonies of reef-building (hermatypic) corals exhibit a wide range of shapes, but well-nigh can be classified within x general forms. Branching corals have branches that as well have (secondary) branches. Digitate corals await like fingers or clumps of cigars and have no secondary branches. Tabular array corals are table-similar structures of fused branches. Elkhorn coral has big, flattened branches. Foliose corals accept broad plate-like portions rising above the substrate. Encrusting corals grow every bit a thin layer against the substrate. Submassive corals have knobs, columns or wedges protruding from an encrusting base. Massive corals are ball-shaped or boulder-similar corals which may be pocket-sized as an egg or large equally a house. Mushroom corals resemble the fastened or unattached tops of mushrooms. Cup corals wait similar egg cups or cups that accept been squashed, elongated or twisted (McManus et al. 1997). While the growth patterns of stony coral colonies are primarily species-specific, a colony's geographic location, environmental factors (east.g., wave action, temperature, light exposure), and the density of surrounding corals may affect and/or alter the shape of the colony as information technology grows (Barnes, R.D. 1987; Barnes, R.S.Yard. and Hughes 1999, Lalli and Parsons, 1995).
In addition to affecting the shape of a colony's growth, environmental factors influence the rates at which various species of corals abound. One of the most significant factors is sunlight. On sunny days, the calcification rates of corals can exist twice every bit fast every bit on cloudy days (Barnes, R.Due south.K. and Hughes, 1999). This is likely a function of the symbiotic zooxanthellae algae, which play a unique role in enhancing the corals' ability to synthesize calcium carbonate. Experiments have shown that rates of calcification slow significantly when zooxanthellae are removed from corals, or when corals are kept in shade or darkness (Lalli and Parsons 1995).
In general, massive corals tend to grow slowly, increasing in size from 0.v cm to ii cm per year. However, under favorable conditions (high light exposure, consistent temperature, moderate wave action), some species can grow as much every bit 4.5 cm per year. In contrast to the massive species, branching colonies tend to grow much faster. Under favorable weather condition, these colonies tin grow vertically by equally much as 10 cm per yr. This fast growth rate is not every bit advantageous every bit it may seem, still. Mechanical constraints limit the maximum size that branching corals can achieve. As they become larger, a heavier load is placed on the relatively modest area attached to the substratum, rendering the colony increasingly unstable. Nether these circumstances, the branches are prone to snapping off during strong wave activity. The reverse is truthful of the massive-shaped corals, which become more stable equally they grow larger (Barnes, R.South.K. and Hughes, 1999).
Where Reefs Exist

Reef-building corals are restricted in their geographic distribution. This is considering the algal-cnidarian symbiotic machinery needs a narrow and consistent ring of ecology conditions to produce the copious quantities of limestone necessary for reef formation. The formation of highly consolidated reefs simply occur where the temperature does non fall below xviii°C for extended periods of time. This specific temperature restriction -18°C- does non, however, apply to the corals themselves. In Japan, where this has been studied in detail, approximately one-half of all coral species occur where the sea temperature regularly falls to 14°C an approximately 25% occur where it falls to 11°C (Veron 2000). Many grow optimally in h2o temperatures between 23° and 29°C, but some tin tolerate temperatures as loftier as 40°C for limited periods of time. Most require very salty (saline) water ranging from 32 to 42 parts per 1000. The water must also be clear to permit high light penetration. The corals' requirement for high low-cal also explains why near reef-building species are restricted to the euphotic (light penetration) zone, approximately lxx grand (Lalli and Parsons, 1995).

The number of species of corals on a reef declines speedily in deeper water. High levels of suspended sediments tin can smother coral colonies, clogging their mouths which can impair feeding. Suspended sediments can besides serve to subtract the depth to which calorie-free can penetrate. In colder regions, murkier waters, or at depths below seventy k, corals may still exist on hard substrates, simply their capacity to secrete limestone is greatly reduced (Barnes, R.D., 1987).
In light of such stringent environmental restrictions, reefs generally are confined to tropical and semitropical waters. The diversity of reef corals, i.e., the number of species, decreases in higher latitudes up to about 30° northward and s, beyond which reef corals are usually not establish. Bermuda, at 32° due north breadth, is an exception to this rule because it lies directly in the path of the Gulf Stream's warming waters (Barnes, R.D., 1987).
Some other factor that seems to bear upon the diversity of reef-building corals is the ocean in which they are located. At least 500 reef-building species are known to exist in the waters of the Indo-Pacific region. In comparison, the Atlantic Ocean contains approximately 62 known species. The fossil record shows that many species once found across the Atlantic, Pacific and Indian Oceans gradually went extinct in the Atlantic, where the affects of ice ages had stiff impacts on the Caribbean wherein most of the Atlantic reefs reside. Following the closure of the seaway betwixt the Caribbean and the Pacific, several species of corals became restricted to the Caribbean (Veron 2000).
(superlative)
The Structure of Coral Reefs

Coral reefs begin to form when free-swimming coral larvae (planulae) adhere to the submerged edges of islands or continents. Every bit the corals grow and expand, reefs take on one of iii major characteristic structures—fringing, barrier or atoll.Fringing reefs, which are the most common, project seaward directly from the shore, forming borders along the shoreline and surrounding islands. Barrier reefs also border shorelines, but at a greater distance. They are separated from their adjacent state mass past a lagoon of open, often deep water. If a fringing reef forms effectually a volcanic isle that subsides completely below sea level while the coral continues to abound upward, an atoll forms. Atolls are normally round or oval, with a fundamental lagoon. Parts of the reef platform may emerge as one or more than islands, and breaks in the reef provide access to the key lagoon (Lalli and Parsons, 1995; Levinton, 1995; Sumich, 1996).
In the 1830s, Charles Darwin distinguished between the 3 main geomorphological categories of reefs, and suggested that fringing reefs, barrier reefs, and atolls were all related stages in the sequence of atoll reef formation.
All 3 reef types—fringing, barrier and atoll—share similarities in their biogeographic profiles.Bottom topography, depth, moving ridge and current forcefulness, light, temperature, and suspended sediments all act to create characteristic horizontal and vertical zones of corals, algae and other species. While these zones vary co-ordinate to the location and type of reef, the major divisions common to nearly reefs, as they move seaward from the shore, are the reef flat, reef crest or algal ridge, buttress zone, and seaward slope.
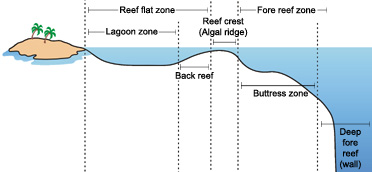
The reef flat, or back reef, is located on the sheltered side of the reef. It extends outward from the shore; and may be highly variable in character. Varying in width from twenty or 30 meters to more than a few yard, the reef flat may range from but a few centimeters to a few meters deep, and big parts may be exposed at low tide. The substrate is formed of coral rock and loose sand. Beds of sea grasses oft develop in the sandy regions, and both encrusting and filamentous algae are common.

Because information technology is so shallow, this surface area experiences the widest variations in temperature and salinity, just it is protected from the full force of breaking waves. Reduced water circulation, the accumulation of sediments, and periods of tidal emersions—when the reef is exposed during depression tide—combine to limit coral growth. Although living corals may be scarce except well-nigh the seaward section of this zone, its many microhabitats support the greatest number of species in the reef ecosystem, with mollusks, worms and decapod crustaceans often dominating the visible macrofauna (Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).

The reef crest, or algal ridge, is the highest signal of the reef, and is exposed at low tide. Lying on the outer side of the reef, it is exposed to the full fury of incoming waves. The width of this zone typically varies from a few, to perhaps 50 m. In this severe habitat, a few species of encrusting calcareous reddish algae flourish, producing new reef material as rapidly equally the waves erode it. Where wave action is severe, living corals are practically nonexistent, but in situations of more moderate moving ridge action, the reef crest tends to be dominated by stoutly branching corals. These closely growing, robust colonies grade ramparts able to withstand the heavy seas. Pocket-size crabs, shrimps, cowries and other animals reside in the labyrinthine subsurface cavities of the reef crest, protected from waves and predators (Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).

The outermost seaward slope (also chosen the fore-reef) extends from the low-tide marker into deep water. Merely below the depression-tide mark to approximately 20 m depth is a rugged zone of spurs, or buttresses, radiating out from the reef. Deep channels that gradient down the reef face are interspersed between the buttresses. These alternating spurs and channels may be several meters wide and up to 300 m long (Barnes, R.D. 1987; Lalli and Parsons, 1995; Sumich, 1996).
The buttress zone serves two master purposes in the reef system. First, it acts to dissipate the tremendous force of unabating waves and stabilizes the reef structure. 2nd, the channels betwixt the buttresses drain debris and sediment off the reef and into deeper water. Massive corals and encrusting coralline algae thrive in this zone of breaking waves, intense sunlight, and abundant oxygen. Minor fish inhabit the many holes and crevices on this portion of the reef, and many larger fish including sharks, jacks, barracudas and tunas patrol the buttresses and grooves in search of food (Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).

Standing downwardly the seaward gradient to about xx yard, optimal light intensity decreases, but reduced moving ridge activeness allows the maximum number of coral species to develop. Beginning at approximately 30 to 40 m, sediments accumulate on the gentle gradient, and corals become patchy in distribution. Sponges, sea whips, ocean fans, and ahermatypic (non-reef-edifice) corals become increasingly arable and gradually replace hermatypic corals in deeper, darker water (Barnes, R.D., 1987; Lalli and Parsons, 1995; Sumich, 1996).
References
Barnes, R.D. 1987. Invertebrate Zoology; Fifth Edition. Fort Worth, TX: Harcourt Brace Jovanovich Higher Publishers. pp. 92-96, 127-134, 149-162.
Barnes, R.Southward.K. and R.N. Hughes. 1999. An Introduction to Marine Ecology; third edition. Oxford, UK: Blackwell Science Ltd. pp. 117-141.
Lalli, C.Chiliad. and T.R. Parsons. 1995. Biological Oceanography: An Introduction. Oxford, UK: Butterworth-Heinemann Ltd. pp. 220-233.
Levinton, J.S. 1995. Marine Biology: Function, Biodiversity, Ecology. New York: Oxford Academy Press, Inc. pp. 306-319.
McManus, J.W., M.C.A. Ablan, S.G. Vergara, B.M. Vallejo, L.A.B. Menez, K.P.K. Reyes, M.L.1000. Gorospe and L. Halmarick, 1997. Reefbase Aquanaut Survey Transmission. ICLARM Educational Series. 18, 61p.
Sumich, J.L. 1996. An Introduction to the Biology of Marine Life, sixth edition. Dubuque, IA: Wm. C. Brownish. pp. 255-269.
Turgeon, D.D. and R.G. Asch. In Press. The State of Coral Reef Ecosystems of the United States and Pacific Freely Associated States. Washington D.C.; NOAA.
Veron, JEN. 2000. Corals of the Globe. Vol 3. Australia: Australian Institute of Marine Sciences and CRR Qld Pty Ltd.
Source: https://www.coris.noaa.gov/about/what_are/
0 Response to "what do animals ranging from corals to humans have in common?"
Post a Comment